What are Dioxins and Furans?
Dioxins and furans are by-products of many industrial processes, including incineration. When waste materials, particularly those containing chlorine (like PVC plastics), are incinerated, these chemicals can be produced.
To prevent or reduce the generation of dioxins and furans, modern incinerators operate at high temperatures and with good combustion efficiency. They also use air pollution control technologies, such as adsorption and hot gas filtration, to capture these and other pollutants. The incineration process is also carefully controlled to ensure complete combustion and minimize the production of dioxins and furans. Proper waste separation to exclude materials that could lead to the production of dioxins and furans is another important preventative measure.
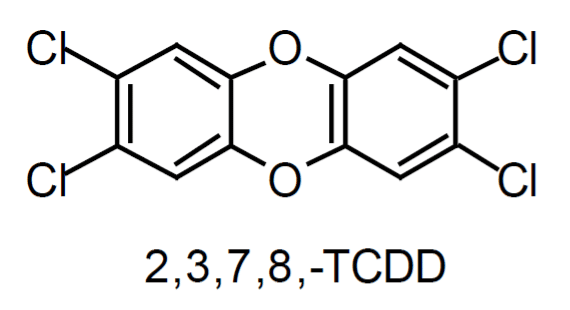
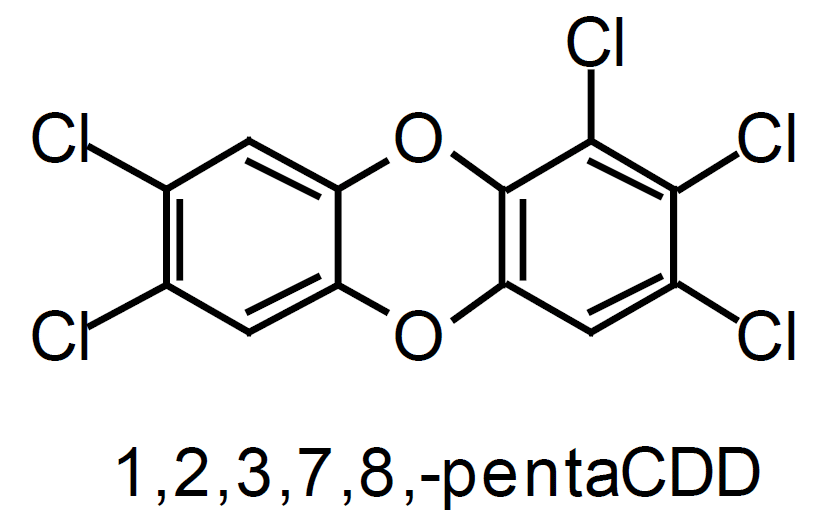
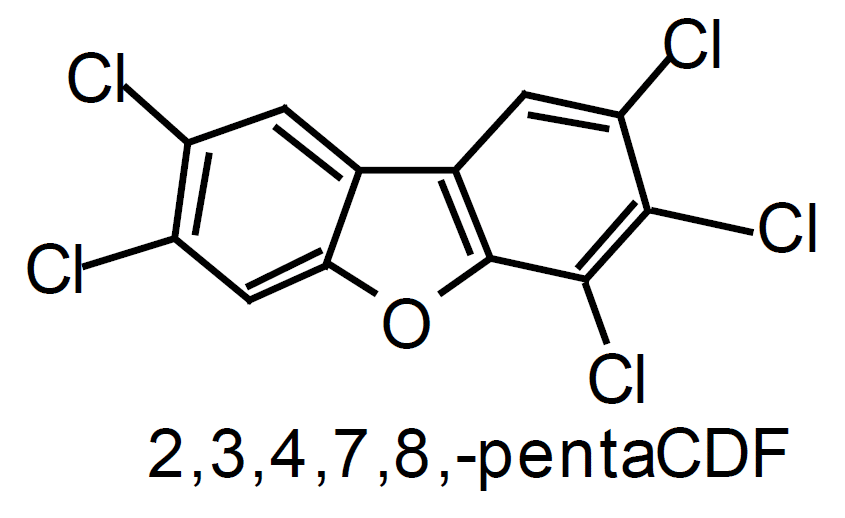
The polychlorinated dibenzo-para-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) are chlorinated, planar, aromatic compounds containing two benzene rings. The terms PCDD/F and dioxins carry the same meaning and are used interchangeably in the literature. The structures of the most toxic forms of dioxin and furan molecules are shown in Figure 1. A dioxin molecule is bonded by two oxygen atoms, and a furan molecule by a single oxygen atom and a direct bond. Under standard atmospheric conditions, all dioxins are solid and are characterised by low vapour pressure and limited solubility in water.
There are 75 different forms of PCDD and 135 different forms of PCDF that are distinguished by the position and number of chlorine atoms attached to the two benzene rings. These different forms of dioxins are called congeners and their number is listed in Table 1. Dioxins and furans with the same number of chlorine atoms constitute a homologue group of isomers. Table 1 shows that there are 16 homologues groups, 8 for PCDD and 8 for PCDF. For example, there are 22 different isomers of dioxins and 38 of furans belonging to the same tetrachlorinated homologue groups.
For toxicity purposes, only the homologues with four or more chlorine atoms are considered and these are called tetra (TCDD, TCDF or D4, F4), penta (PCDD, PCDF or D5, F5), hexa (HxCDD, HxCDF or D6, F6), hepta (HpCDD, HpCDF or D7, F7) and octa (OCDD, OCDF or D8, F8). It is these 10 PCDD/F homologues that are normally quoted in the literature. Note the overlapping terminology as the penta homologues have the same abbreviation (PCDD, PCDF) as all 210 dioxins and furans (PCDD/F).
Those PCDD/F that have four chlorine atoms substituted in positions 2, 3, 7, and 8 are considered to be the most toxic. Their toxicity depends on the location and the number of additional chlorine atoms attached to the benzene rings. The 17 most toxic PCDD/F isomers, belong to the 2, 3, 7, 8-subsituted group. For comparison, the LD 50/30 values of some toxic (2, 3, 7, 8-substituted) and non-toxic dioxins are listed in Table 2 (Steel Times, 1995). LD 50/30 represents a lethal dose of a chemical to 50% of test animals after 30 days. In general, the more chlorine atoms a PCDD/F molecule has, the less toxic it becomes.
Since the toxicity of a dioxin congener depends on the level of chlorination and location of chlorine atoms on the benzene rings, three toxicity equivalence schemes, called Eadon, Nordic and ITEQ (international toxic equivalent, also denoted as NATO or CCMS for NATO Committee on the Challenges of Modern Society, 1988) have been developed. Within each scheme, 2, 3, 7, 8-substituted PCDD/F have weighting or conversion factors, as illustrated in Table 3. These factors are applied to calculate the equivalent concentration of a dioxin congener. Note a small difference between NATO and Nordic schemes, which differ only in the weighting factor assigned to 1, 2, 3, 7, 8 PCDF. Recent studies indicate good correspondence between the chemical concentration of PCDD/F, expressed in equivalent concentration, and the biological potency of PCDD/F (eg Kopponen et al 1994, Clemons et al 1997).
The ITEQs are calculated as follows. If, for example, the concentration of 2, 3, 4, 7, 8 PCDF is 0.1μg/m3, its ITEQ value is (0.1×0.5) 0.05μg/m3, where the factor 0.5 was extracted from Table 3. Similarly, the concentration of other dioxins needs to be weighted according to the conversion factors listed in Table 3. The sum of the converted concentrations is then reported as an ITEQ value. In the dioxin literature, the ITEQ concentration is the one most often quoted, followed by the total PCDD/F concentration, and the Nordic-equivalence concentration. As a rule of thumb, ITEQ values are smaller by about 50 times than PCDD/F concentration values. So, one can divide PCDD/F concentration by 50 to obtain a very approximate but fast estimation of ITEQ concentration, if the latter values are not available.
It is generally accepted that the mole fractions of dioxin congeners and tetra to octa homologues are specific to a dioxin formation process. The relative concentration of homologues and congeners, usually plotted as a histogram, is called the dioxin signature or dioxin fingerprint. The complete signature contains 10 homologues, up to 17 toxic congeners (as listed in Table 3), and the relative ratio of the sum of congeners to the total PCDD/F. Usually, only a subset of the 17 congeners is included in the signature and even then some of the congeners may be lumped into one number, eg all hexachlorinated congeners are reported together as one item in the signature.
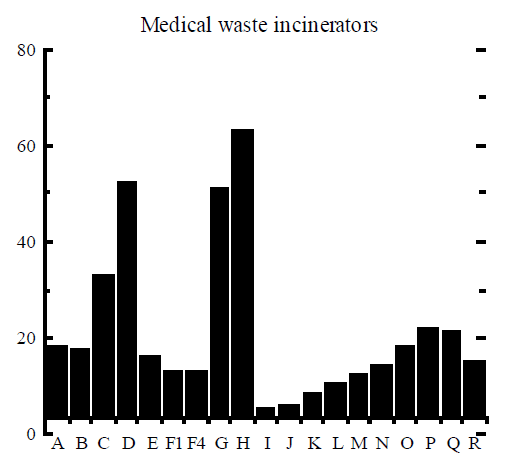
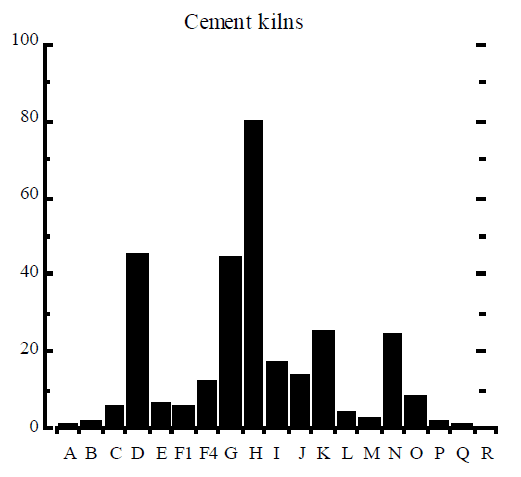
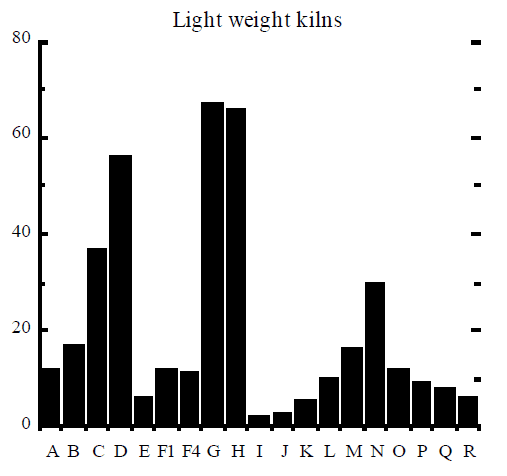
Rigo et al (1995) assembled signatures of dioxin formation for a range of industrial processes and these signatures are reproduced here in Figure 2. The figure contains the relative mole fractions of 2, 3, 7, 8 congener to homologue (9 bars) and the mole fraction of homologue to the total PCDD/F (10 bars). The example of TCDD (“A” bar in Figure 2) can be used to illustrate how the signature was obtained. The amount of 2, 3, 7, 8 TCDD (in moles) was divided by the total amount of all 22 homologues of TCDD, to obtain a ratio which was then multiplied by 100% and plotted in Figure 2. Similarly, the amount of all 22 tetrachlorinated dioxins was divided by the total amount of PCDD/F in moles, multiplied by 100%, and plotted as “I” bar in Figure 2.
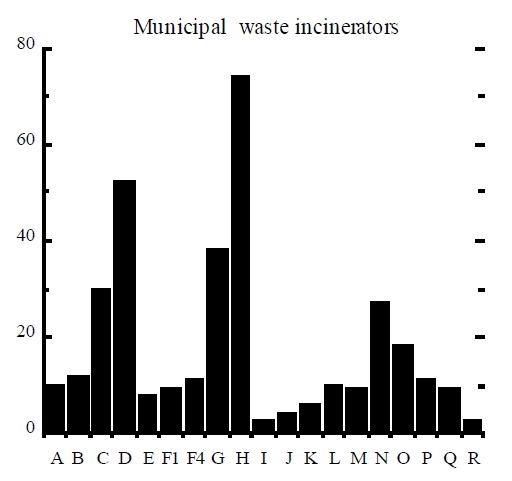
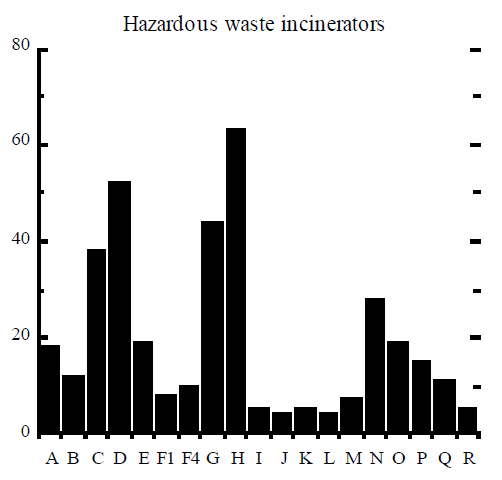
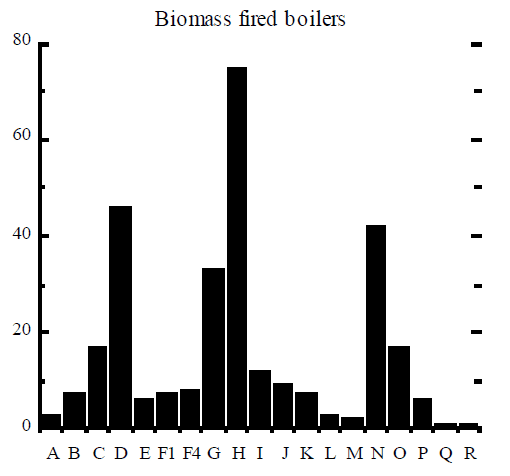
A good example of different dioxin signatures is observed in stack gas emissions from boilers used in the pulp and paper industry (Luthe et al, 1997). Two types of boilers are employed, recovery boilers that burn the so-called black liquor solids containing chlorine from the Kraft process, and power boilers that burn wood waste that often contains chlorine salts, especially in coastal areas. Emissions from recovery boilers tend to contain mostly dioxins, whereas emissions from power boilers show approximately equal concentrations of dioxins and furans.
Another excellent example illustrating the utility of dioxin fingerprint for the identification of the PCDD/F source comes from the province of British Columbia in Canada. Kraft pulp mills operating there employed a bleaching technology, which used chlorine gas. This led to the formation of dioxins, and their subsequent emission in discharge water. The dioxin signature from Kraft pulp mills was dominated by three tetrachlorinated isomers. The problem was recognised and the chlorine gas was replaced by chlorine dioxide or nonchlorinated reagents (Rappe 1996), resulting in dioxin-free paper bleaching. However, the routine testing of the sediment in the vicinity of effluent discharge points from the pulping mills soon disclosed evidence of new dioxin deposition, which had a different signature (dominated by hexachlorinated dioxins laid over a typical pulp-bleaching fingerprint) from that generated by gaseous schlorine bleaching.
It was speculated that the dioxin source was located in the paper plant but the source would have to be different from the bleaching process. Careful investigation soon revealed that on occasions the mills accepted wood chips that might have been obtained from lumber contaminated with polychlorinated phenols (PCP). In the lumber industry, PCP were applied to prevent staining of undried wood with sap. The testing program that followed narrowed down the source of dioxin to polychlorinated phenoxyphenols, an impurity which was originally present in PCP used to treat the wood (Luther et al, 1993). At the same time, a similar investigation using the analysis of dioxin fingerprint concluded that the recycled dioxin-contaminated corrugated containers caused the dioxin emission problems from other Canadian pulp mills (Berry et al, 1993).
Macrotec proposes the use of flue gas abatement systems with activated carbon scrubbing for the removal of dioxins and furans. Advanced incinerators with proper combustion control, and the control of temperatures before and after scrubbing also reduces the formation of dioxins and furans.