What is Incineration?
Incineration is a waste management technique used to treat municipal, biosolids, hazardous and non-hazardous industrial and medical wastes, and other liquids, residues, and toxic and flammable gases released into the environment unacceptable. Incineration converts these wastes into the flue gas, ash, and heat, with the ash being mostly inorganic substances that are non-combustible.

For some wastes, such as the incineration of fumes, ash-free liquids, or solids, incineration can be called disposal. But for most solid or liquid wastes, incineration will only be one step in treating the waste, with residues remaining for subsequent disposal. It is important to note that only combustible substances will be incinerated. Non-combustible substances will report to either the ash or flue gas depending on the boiling point of the substance.
Incineration offers the following potential advantages:
- Volume reduction
- Detoxification
- Environmental impact mitigation
- Regulatory compliance
- Energy recovery
Combustion Kinetics
Incineration is a combustion reaction, which involves several chemical reactions. This combustion process is dependent on the temperature, concentration of the reacting substances, and in some cases, the static pressure. Combustion kinetics explain how these relationships affect the reaction rate. What makes incineration different from most combustion processes is that the primary goal is to achieve 100% reaction completeness, i.e. complete destruction of the waste feed, and not the release of energy. This is especially important for the incineration of hazardous wastes.
The most important reactions to take note of is the oxidation of Carbons and Hydrocarbons to form flue gas; the oxidation of Carbon Monoxide; the formation of Nitrogen Oxides, Sulphur Oxides and Halogens and their acids.
When waste is loaded into an incinerator, the initial reaction is the combustion of that waste, or more specifically the oxidation of the carbons and hydrocarbons to form flue gas and ash. The reaction of Carbons and Hydrocarbons require Oxygen and will only start at the ignition temperature of that waste. Moisture content (how much water is in the waste) will also affect the reaction rate, as the water must also be evaporated (boiled). This is an endothermic reaction, as the liquid molecules must adsorb heat to transform into gas molecules. Thus, moisture content will also affect the amount of heat released by the reaction and the amount of auxiliary fuel required.
Carbon Monoxide
Carbon monoxide is odourless, invisible, poisonous gas, and an important air pollutant. Carbon monoxide is produced by incomplete combustion, often near the incinerator hearth where wasted is densely packed in low Oxygen areas, or as an intermediate combustion product. The kinetics of Carbon monoxide have been well studied, and Figure 1 indicates the relationship between temperature and the reaction rate.

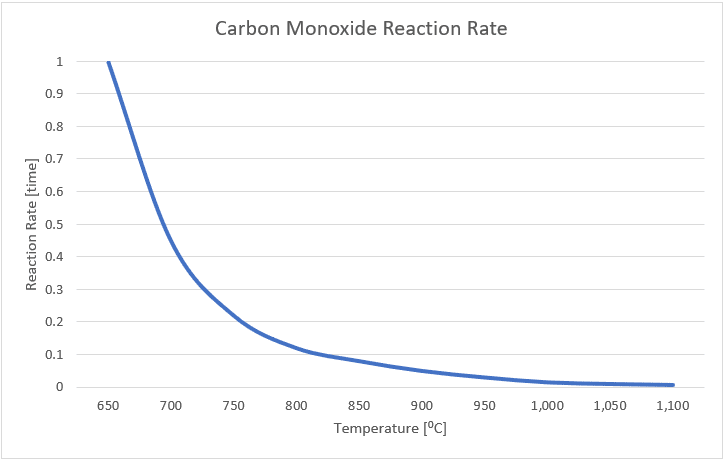
FIGURE 1: Reaction rate of Carbon Monoxide
For complete combustion (of Carbon Monoxide and other substances), one often hears of time, temperature, and turbulence. Unfortunately, the flue gas streams from actual incinerators does not have a single unique time nor temperature characterising their history in the system. It is likely that most of the unoxidized substances is not a residual “heel” of an overall reaction, but rather the unreacted substances of packets of flue gas that never made it to satisfactory combustion conditions. These “failure modes” are not always easily identifiable, as the mean temperature, oxygen concentration, and residence time might all be satisfactory. However, some fraction of the system flow does not achieve the critical parameter thresholds that assure burnout. Generally, if only 99% destruction of a feed material is achieved, it is not because 100% of the flow is 99% burned, but more likely, that 99% of the flow is fully burned out and 1% did not burn at all. One can picture the total flow through an incinerator as a composite of a large number of small, individual packets of gas each with its own unique composition and time–temperature history. Failure to achieve complete combustion could be due to a fraction of the packets flowing where:
- The incinerator fails to mix sufficient air with the combustible.
- The system mixes sufficient air for combustion to be completed, but the passage of the packet across cold surfaces keeps or brings the temperature below the ignition threshold where fast oxidation reactions occur.
- The system mixes an excessive quantity of air that, through dilution, brings the temperature below the ignition threshold, where fast oxidation reactions occur.
- The system includes flow paths such that some packets of gas, otherwise able to burn, move too quickly through the hot regions of the furnace and are quenched below combustion temperatures before oxidising.
Nitrogen Oxides (NOx)
Nitrogen Oxides (NOx) represents a group of seven compounds, with NO₂ normally acting as a surrogate for the group as it is the most prevalent form of NOx. NOx is an important group of highly reactive gasses, and important pollutants. NO₂ is not only an important pollutant by itself, but also reacts in the atmosphere to form ozone (O₃) and acid rain.
In combustion systems, nitrogen oxides arise through fixation of nitrogen from the combustion air with oxygen (thermal generation). Also, NOx is formed by oxidation of nitrogen entering the system bound in the fuel (fuel nitrogen generation). At very high temperatures, the dominant source of NOx is thermal generation but, at lower temperatures, fuel nitrogen mechanisms dominate.
NOx is formed in combustion systems in one of three ways:
- Thermal NOx – is usually formed above 1,400⁰C, and formation increases significantly with increased temperatures. The formation is typically controlled by reducing the peak and average flame temperatures.
- Fuel NOx – formed by the oxidation of nitrogen entering the system bound in the fuel.
- Prompt NOx – formed from molecular nitrogen in the air combining with fuel in fuel-rich conditions which exist, to some extent, in all combustion. This nitrogen then oxidizes along with the fuel and becomes NOx during combustion, just like fuel NOx.
NOx abatement and control technology is a relatively complex issue and is broadly defined as either a Pollution Prevention Method or an add-on Abatement Technology.
Sulphur Oxides
Many waste streams and fossil fuels contain sulphur. The sulphur can be present in any or all of its many oxidation states from S−2 to S+6. Of particular interest from an air pollution standpoint is the sulphur appearing as organic or inorganic (pyritic) sulphur, free sulphur, or sulphur appearing in organic or inorganic acid forms. In each of these cases, sulphur can be expected to appear in the fuel gases as sulphur dioxide or trioxide. A portion of the sulphur that exists as inorganic sulphates in fuels or waste materials such as gypsum-filled wallboard (calcium sulphate) may be released by reduction reactions, especially in the high-temperature environment on the grate in mass-burning incinerators.
Sulphur oxides have importance as a pollutant owing to their health effects (especially in combination with respirable particulate matter) and their corrosive effects on natural and man-made materials. In the combustion system, sulphur trioxide reacts with water vapour to form sulfuric acid, which has a dew point considerably above that of pure water.
See our Guide on Incinerator Flue Gas Abatement for more info.
Halogens and Their Acids
Halogens are important constituents of waste. Some of the organic compounds of the halogens are toxic, carcinogenic, or otherwise of concern regarding their health effects. Further, the acid gases formed during combustion are strong acids that can attack metals (e.g., in boilers) and can be highly corrosive in their water solutions in scrubbers. Chlorine and hydrochloric acid are generally the most important members of this family, although fluorine, bromine, iodine, and their acids can be more problematic in system design, operations, and pollutant control.
Chlorine appears in waste streams both in inorganic salts (e.g., sodium chloride) and in organic compounds. In the combustion of many industrial wastes and, importantly, in MSWs, a substantial quantity of organic matter containing chlorine may be charged to the furnace. In the combustion environment (usually containing hydrogen in considerable excess relative to the chlorine) the organic chlorine is converted, almost quantitatively, to hydrogen chloride (hydrochloric acid). In mass-burning incinerators, about 35–40% of the stoichiometric HCl generated through combustion is absorbed by alkaline constituents of the ash (Na2O, CaO, etc.).
